We all experience the effects of aging – muscles weaken, skin loses elasticity, and our bodies just don't recover as quickly as they used to. A key reason for these age-related changes lies within our very own building blocks: somatic stem cells. These remarkable cells are the unsung heroes responsible for maintaining and repairing our …
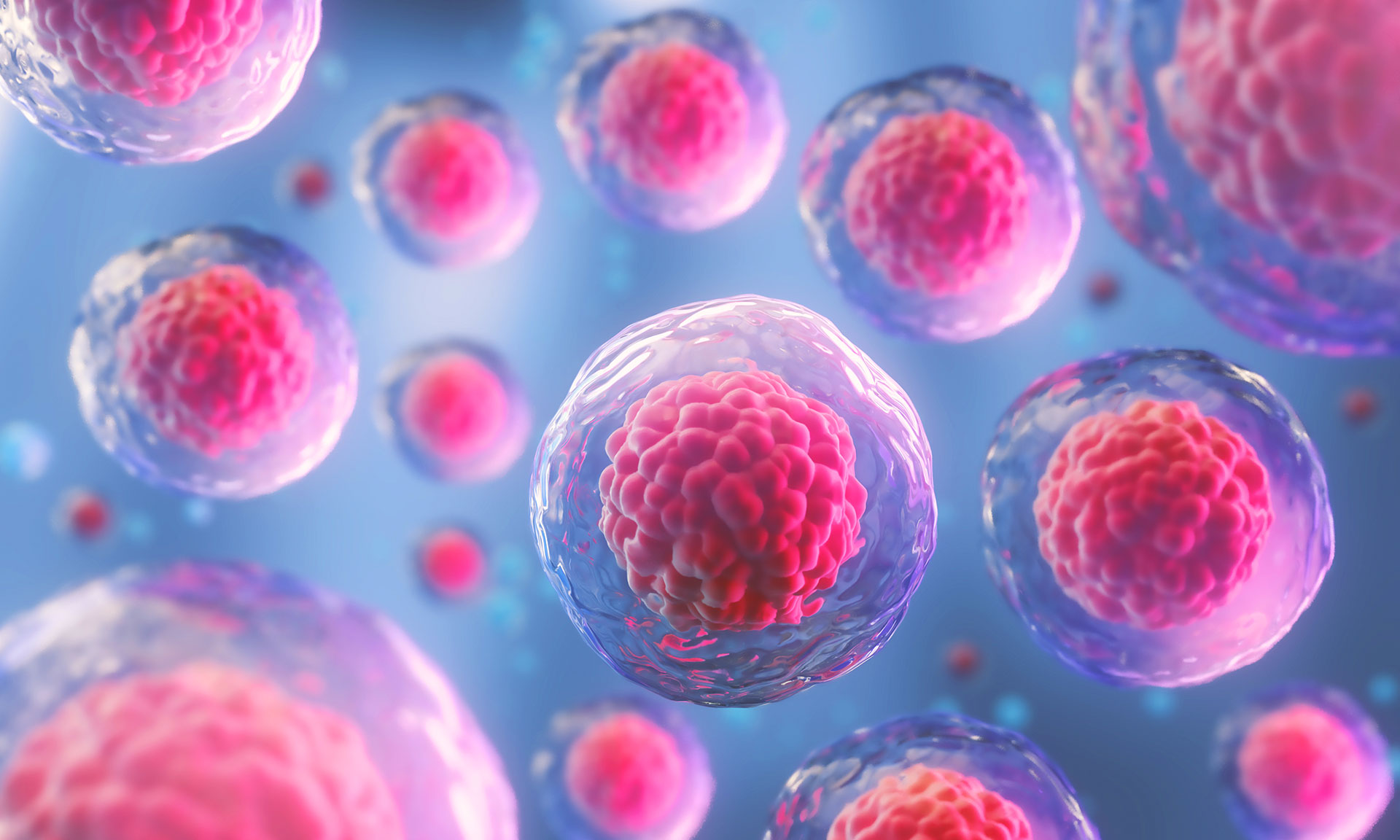
We all experience the effects of aging – muscles weaken, skin loses elasticity, and our bodies just don’t recover as quickly as they used to. A key reason for these age-related changes lies within our very own building blocks: somatic stem cells. These remarkable cells are the unsung heroes responsible for maintaining and repairing our tissues throughout life. However, as we age, these stem cells gradually lose their ability to perform their vital roles.
A comprehensive review by leading researchers Thomas A. Rando, Anne Brunet, and Margaret A. Goodell delves into the “hallmarks of stem cell aging,” outlining five key characteristics that change in these critical cells with time. Understanding these changes is crucial because they not only shed light on the aging process itself but also offer promising targets for future therapies aimed at keeping us healthier, longer.
The Unsung Heroes: What Do Stem Cells Do?
Think of somatic stem cells as your body’s personal repair crew. They reside in various tissues, like your muscles, brain, skin, and bone marrow. Their two main jobs are:
- Tissue Homeostasis: Continuously replacing old, worn-out cells to keep tissues healthy and functioning normally (e.g., refreshing your skin cells).
- Tissue Regeneration: Springing into action after an injury or disease to repair damage and restore function (e.g., healing a cut or mending a broken bone).
While stem cells are generally more protected than other cells from some aging mechanisms, they are still vulnerable to the passage of time and various stressors. This vulnerability leads to their decline, which is a major contributor to the overall aging of our bodies.
The Five Hallmarks of Aged Stem Cells
Instead of focusing on molecular changes that vary widely across different stem cell types, the researchers highlighted five functional hallmarks that are central to how stem cells perform their crucial roles:
- Changes in Depth of Quiescence: Most stem cells spend much of their time in a “resting” or quiescent state, rarely dividing. This low-activity state helps them preserve themselves over a lifetime. With age, however, this balance can be disrupted. Some aged stem cells might enter a deeper state of quiescence, making them slower to activate when needed for repair. Conversely, others might enter a shallower state, becoming more prone to dividing unnecessarily, which can lead to their eventual depletion. For example, in muscle stem cells, both “too deep” and “too shallow” quiescence can impair muscle regeneration.
- Changes in Self-Renewal Propensity: Self-renewal is the ability of a stem cell to divide and create more stem cells, ensuring the pool of repair cells is maintained. In aging, this process can go awry. For instance, hematopoietic stem cells (HSCs), which give rise to all blood cells, show an increased tendency to self-renew at the expense of differentiating into specialized blood cells. While this might seem like a good thing (more stem cells!), it can lead to an imbalanced production of blood cells, contributing to age-related blood disorders.
- Altered Cell Fate of Progeny: Stem cells differentiate into specific cell types to repair tissues. With age, this differentiation process can become skewed or aberrant.
- Skewed Differentiation: For example, in the brain, neural stem cells in older individuals tend to produce more astrocytes (support cells) instead of new neurons, contributing to a decline in brain function.
- Abnormal Cell Fates: In muscle, aged muscle stem cells can mistakenly turn into fat or fibrous tissue instead of muscle, leading to muscle weakness and scar formation. This is a “gain of function” that is detrimental.
- Malignant Transformation: Critically, as stem cells accumulate mutations over time, they can contribute to the development of cancer, which is largely a disease of aging.
- Altered Resilience (Survivability): Resilience refers to a cell’s ability to withstand stress and avoid death. Aged stem cells often show a loss of resilience, meaning they are more likely to die in response to everyday stresses, injuries, or even during necessary processes like cell division (a phenomenon called “mitotic catastrophe”). This contributes to a dwindling pool of active stem cells and impairs the body’s ability to recover.
- Changes in Population Heterogeneity: Healthy stem cell populations are diverse; they contain different “subtypes” of stem cells that can respond to various needs. With age, this heterogeneity (diversity) can be lost, as certain stem cell subtypes might expand at the expense of others. This reduced diversity can limit the overall adaptability and regenerative capacity of the stem cell pool.
Looking Ahead: Rejuvenating Our Future
These five hallmarks paint a clear picture of how stem cells age, driven by both intrinsic changes within the cells themselves (like accumulated DNA damage) and extrinsic influences from their surrounding environment (like increased inflammation in aged tissues).
The good news? By identifying these specific functional changes, researchers are gaining valuable insights into potential targets for therapeutic strategies. The goal is not just to extend lifespan but to extend “health span” – the period of life spent in good health and free from age-related diseases. By finding ways to rejuvenate our stem cells, we could potentially boost our body’s natural repair mechanisms, leading to better tissue function, faster recovery, and a more vibrant life as we age.
What aspect of stem cell aging do you find most interesting or concerning?
Hallmarks of stem cell aging
Rando, Thomas A. et al.
Cell Stem Cell, Volume 32, Issue 7, 1038 – 1054